What Does the Latent Heat of Vaporization Represent
Since the heat of vaporization added during vaporization is not directly noticeable in an increase in temperature but can nevertheless be found in the vaporized substance in the form of internal energy the heat of vaporization is also referred to as latent heat. See answer 1 Best Answer.
Latent Heat Definition Types Formula Fusion And Vaporization
What Does The Latent Heat Of Vaporization MeasureThe latent heat of vaporization is the amount of heat energy that has to be added to a liquid at the boiling point to vaporize it.
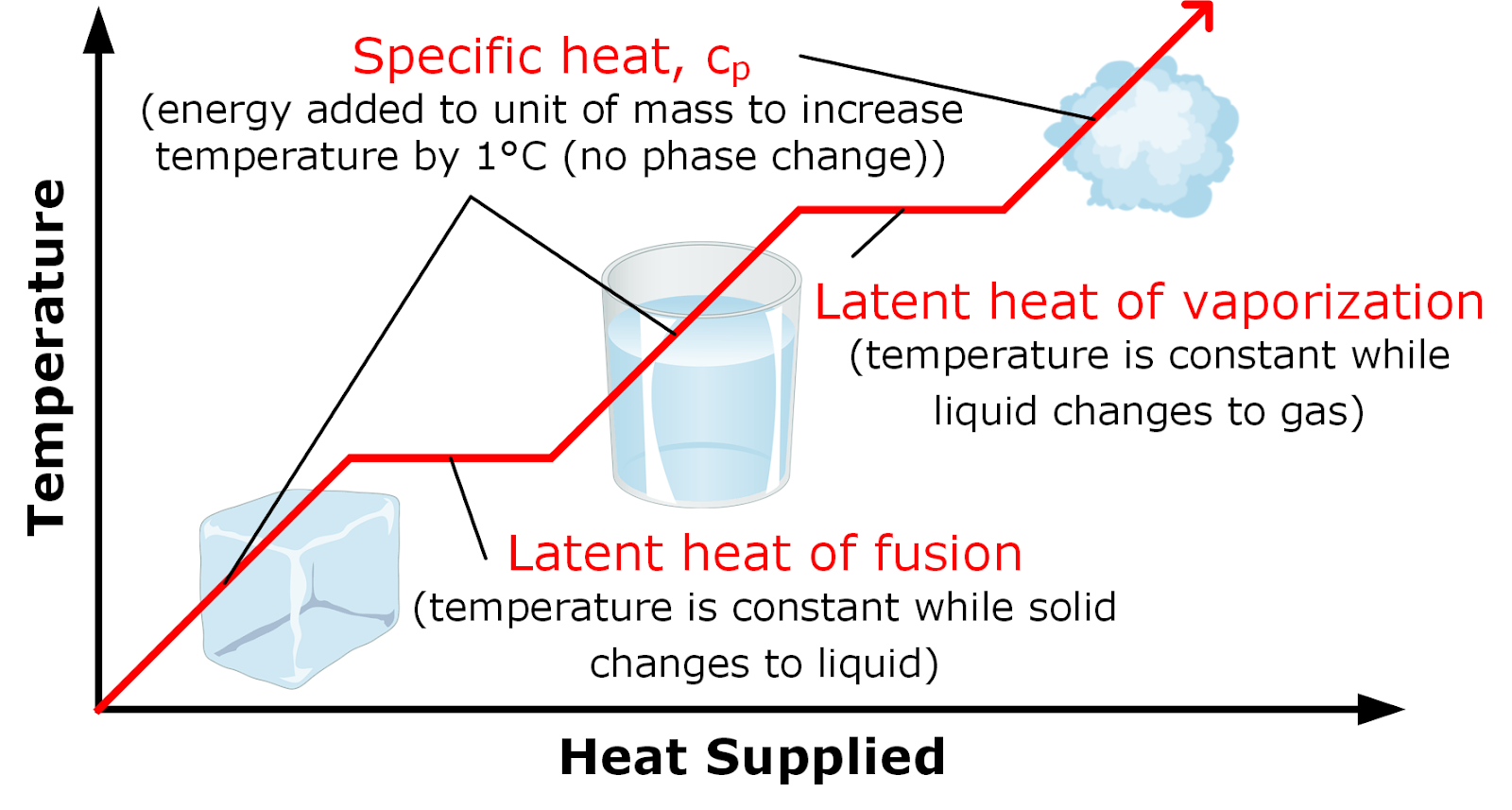
. The heat of vaporization is characterized as the amount of energy heat required to convert 1g of a liquid into a gas without any change in the temperature of the liquid itself. 2nd- latent heat of fusion Heat required to change a unit mass of water from solid to liquid and vice - versa. This is the amount of energy require to.
Phase changes are generally considered at constant rather than constant volume. As with the freezing point the temperature of the remaining liquid water stays the same during the evaporation process. Latent originates from the Latin word latere which intends to lie covered up or hid.
In this case the latent heat of vaporization L v is equivalent to 2501 kJkg. The heat is called latent because it does not heat up the liquidMar 23 2018What is latent heat of vaporization and i. The latent heat of condensation is always negative since heat is released by the substance whereas latent heat of vaporization is always positive since heat is absorbed by the substance.
The latent heat of vaporization is what is commonly referred to as boiling. What does it mean by latent heat of vaporization of water 2256 x 106Jkg. The heat of vaporization.
If conditions allow the formation of vapour bubbles within a liquid the vaporization process is called boiling. The energy absorbed in this process is called heat of vaporization. What Does Vaporization Mean In Sciencevaporization conversion of a substance from the liquid or solid phase into the gaseous vapour phase.
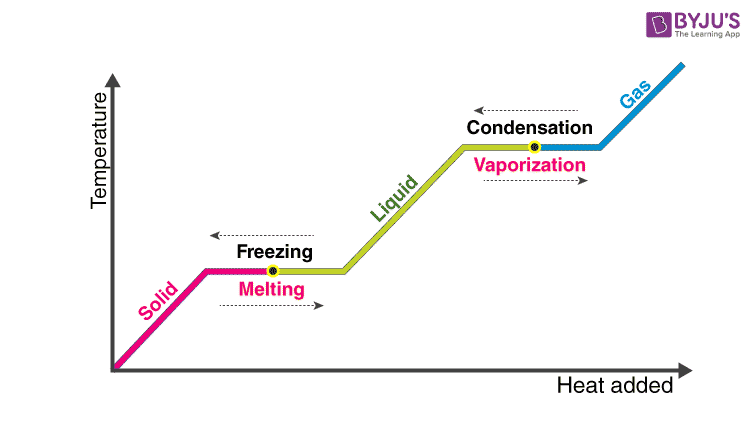
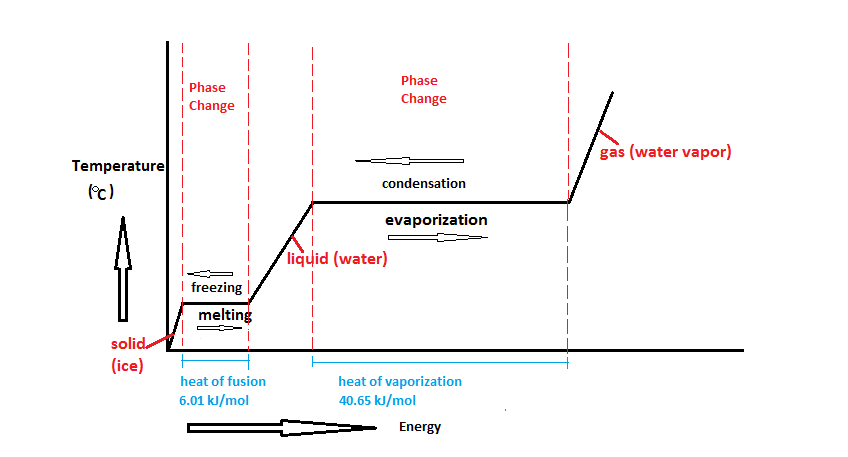
Therefore like the heat of fusion the latent heat of vaporization measures the heat given to a liquid in order to change its state into its gaseous state. What is the latent heat vaporization. Direct conversion from solid to vapour is c.
When latent heat is added no temperature change occurs. Latent heat is the amount of heat added to or removed from a substance to produce a change in phase. When a material in liquid state is given energy it changes its phase from liquid to vapour without change in temperature the energy absorbed in the process is called latent heat of vaporization.
This is because a huge amount of energyrelative to meltingis required to vaporize the puddle. October 29 2021 Nora FAQ. J also known as the latent heat of vaporization or heat of evaporation.
The latent heat of vaporization of water is 540 calgC. Hence a more complete equation to calculate the heat of vaporization is. The latent heat of vaporization of water is about 2260kJKg which is equal to 408kJmol.
In order to understand the temperature heat and thermal equilibrium you are planning to do an interesting experiment in the lab. The term latent comes from Latin and means to be hidden or not to appear directly. The enthalpy of vaporization ΔHv is additionally named the latent heat of vaporization.
Latent comes from the Latin latere which means to lie hidden or concealed. Latent heat is the additional heat required to change the state of a substance from solid to liquid at its melting point or from liquid to gas at its boiling point after the temperature of the substance has reached either of these points. The heat of vaporization is latent heat.
1st- latent heat of vaporization Heat required to change the phase of water from liquid to gas and gas to liquid without changing its temperature. Latent heat is the additional heat required to change the state of a substance from solid to liquid at its melting point or from liquid to gas at its boiling point after the temperature of the substance has reached either of. The latent heat of vaporization is what is commonly referred to as boiling.
Latent heat is the extra heat required to change the condition of a substance from solid to fluid at its softening point or from fluid to gas at its breaking point after the temperature of the substance has come to both of these focuses. You are trying to cool a 12 kg piece of iron which you heated initially at 650C. Latent heat of vapourisation is a physical property of a substance.
This energy breaks down the intermolecular attractive forces. Thus when the process of evaporation is complete the former 1 kg. The heat of vaporization is a latent heat.
You started pouring 15C water over it. What is the latent heat of vaporization. Latent heat of vaporization is a physical property of a substance.
Where ΔU vap is the difference in internal energy between the vapor phase and the liquid phase ΔU vap H vapor H liquid and pΔV is the work done against the ambient pressure. The enthalpy of vaporization is a function of. When a material in liquid state is given energy it changes its phase from liquid to vapor.
The energy required to boil a substance. Heat of Vaporization of Water. What does latent mean in chemistry.
Latent comes from the Latin latere which means to lie hidden or concealed. And ΔHv is the distinction between the enthalpy of the soaked fume and that of the immersed fluid at a similar temperature. ΔH vap ΔU vap pΔV.
Water has high specific heat. This energy breaks down the intermolecular attractive forces and also must provide the energy necessary to expand the gas the pΔV work. The heat of vaporization is a latent heat.
Latent heat of vaporization is actually the total amount of enthalpy a kind of heat necessary to accomplish a phase change for a liquid or gas at the boilingcondensation point. This is the amount of energy require to. What does the latent heat of vaporization measure.
In case of liquid to gas phase change this amount of energy is known as the enthalpy of vaporization symbol H vap. Latent heat is the amount of heat added to or removed from a substance to produce a change in phase. Latent heat of vaporization is the heat consumed or discharged when matter disintegrates changing stage from fluid to gas stage at a consistent temperature.

Want To Unlock The Mystery Surrounding The Properties Of Air Then Check Out Our Post On The Psychrometric Chart Diy และงานฝ ม อ
No comments for "What Does the Latent Heat of Vaporization Represent"
Post a Comment